롤링 뉴스(10 개)
-
2024학년도 후기 정보기술대학원 신입생 모집
더보기 -
2024학년도 2학기 전임교원 공개채용
더보기 -
2024년도 제9회 인천대 문학상 공모
더보기 -
2024학년도 신입생 기초학습능력 진단, 증진 프로그램 운영 안내
더보기 -
인천대학교 법학부 2024학년도 1학기 전공 진로 특강
더보기 -
대학생활상담센터 심리상담 후기 모음
더보기 -
학산도서관 독서문화행사 1분기 테마도서전 「첫 문장의 힘」
더보기 -
2024년 대학 구성원 참여형 INU 콘텐츠 공모전 실시 안내
더보기 -
[2024 한국초등영어교육학회ㆍ한국중등영어교육학회 공동 봄 학술 세미나] AI 디지털 시대의 영어 교수학습 및 평가
더보기 -
2024년 상반기 테마인문고전특강
더보기
완벽한 교육으로 품격있는 학생을 완성합니다.
국립인천대학교의 생생한 뉴스를 전합니다.
-
 인천대, 유료 가족회사 현판 전달식 및 간담회 성료
인천대, 유료 가족회사 현판 전달식 및 간담회 성료지난 19일(금), 인천대 LINC3.0사업단(단장 김규원)은 INU이노베이션센터에서 「유료 가족회사 현판 전달식 및 간담회」를 개최하였다. 유료 가족회사 제도는 지역 기업과 대학
2024.04.23 -
 인천대학교, 지구의 날 기념 캠페인 실시
인천대학교, 지구의 날 기념 캠페인 실시2024년 4월 22일, 인천대학교에서는 환경보호의 중요성을 강조하고 지구의 소중함을 되새기기 위해 지구의 날을 기념하는 특별한 행사를 개최했다. 이번 행사는 '지구의 날을 기념한
2024.04.23 -
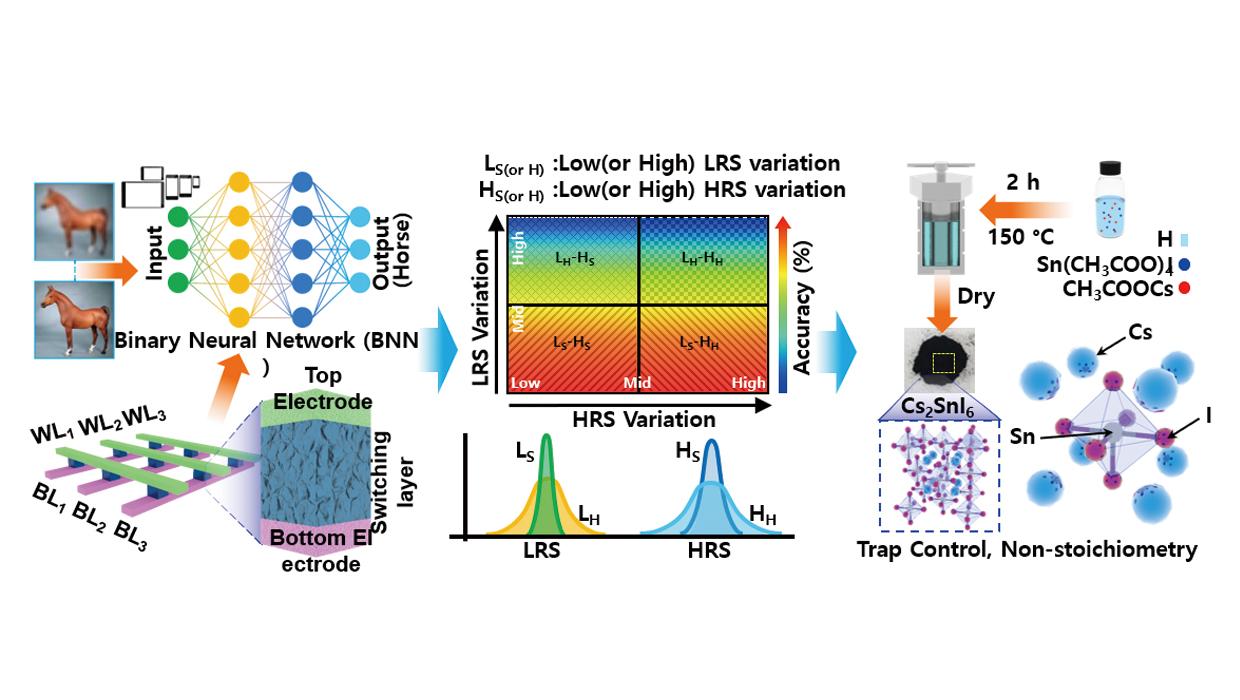 인천대 진성훈교수-한양대학교 김형진 교수 공동연구팀 “차세대 인공지능을 위한 환경 친화적 메모리 솔루션”개발
인천대 진성훈교수-한양대학교 김형진 교수 공동연구팀 “차세대 인공지능을 위한 환경 친화적 메모리 솔루션”개발인천대 진성훈교수 공동연구팀은 차세대 메모리 기술로 주목받는 세슘주석요오드 Cs2SnI6 (CSI) 기반의 저항변화 메모리(RRAM) 소자가 이진 신경망(Binary Neural
2024.04.23 -
 인천대학교 법학부 전공 진로특강 4번 타자, 유승민 전 국회의원 출격한다
인천대학교 법학부 전공 진로특강 4번 타자, 유승민 전 국회의원 출격한다제22대 총선 후 첫 대학 특강, 그가 생각하는 청년제22대 총선 후 첫 대학 특강, 그가 생각하는 청년의 미래와 정치는 무엇일까? 제가 꿈꾸는 보수는 정의롭고 공정하며, 진실되고
2024.04.23 -
 인천대학교 인문대학 지역사회 지식나눔 인문학 특강 개최
인천대학교 인문대학 지역사회 지식나눔 인문학 특강 개최인천대학교 인문대학과 학산도서관이 공동으로 시민과 학생, 교직원을 대상으로 오는 4월 25일~5월 16일 기간 중 총 4회에 걸쳐 매주 목요일(15:00~17:00)에 대면으로 인
2024.04.22 -
 인천대 학산도서관,‘2024 북-페스타: 도서관에서 만나는 미래’성료
인천대 학산도서관,‘2024 북-페스타: 도서관에서 만나는 미래’성료국립 인천대학교(총장 박종태) 학산도서관은 지난 8일~18일 인천대학교 문헌정보학과 16대 학생회 LI-NK와 협력하여 운영한 2024 북-페스타 행사가 성황리에 종료되었다고 알
2024.04.22 -
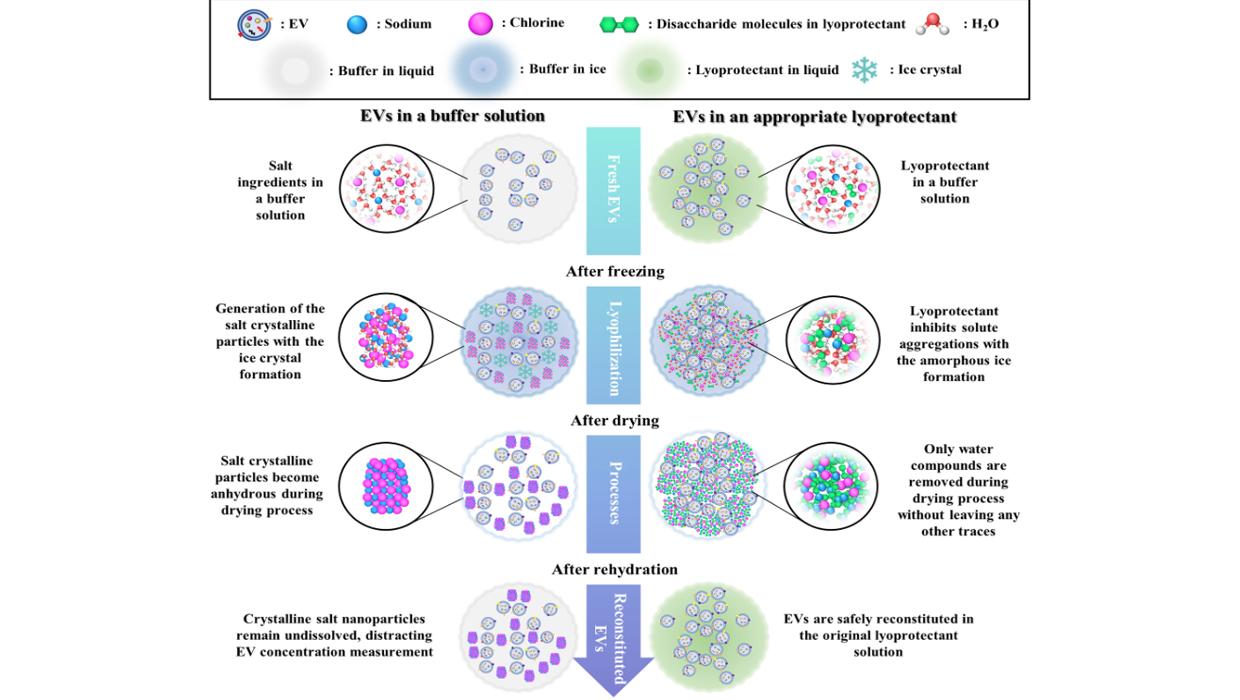 인천대 바이오-로봇시스템공학과 차재민 교수팀 세포외소포체의 동결건조에 특화된 동결건조보호제를 활용한 동결건조공정 개발
인천대 바이오-로봇시스템공학과 차재민 교수팀 세포외소포체의 동결건조에 특화된 동결건조보호제를 활용한 동결건조공정 개발인천대학교 바이오-로봇시스템공학과 조직공학실험실 (지도교수: 차재민) 연구진이 세포외소포체의 동결건조에 특화된 동결건조보호제 조성을 최적화하고 이를 활용한 동결건조공정을 개발했다고
2024.04.22 -
 인천대학교 사범대 수학교육과와 인천어린이과학관 간 MOU 체결
인천대학교 사범대 수학교육과와 인천어린이과학관 간 MOU 체결인천대학교 수학교육과는 지난 18일 인천어린이과학관과 상호협력 MOU를 체결했다고 밝혔다. 협약식은 인천어린이과학관에서 진행되었으며 김석철 인천대 사무처장, 이동선 수학교육과 학과
2024.04.19
SNS(4 개)
#세계로도약하는 #국립인천대학교 # 미래의리더
#잠재력실현 #지역을넘어서세계로
-
국가근로장학금
23 2024.04[사범대학] 2024학년도 1학기 사범대학 국가근로장학생 추가 모집 안내(~4/29(월))
2024학년도 1학기 사범대학 국가근로장학생을 모집합니다.(1) 모집인원 : 1명(2) 근
-
모집
23 2024.04[창업지원단]제 6차 인천시 스포츠산업 창업지원실 입주기업 모집 공고
인천대학교 창업지원단 공고 제2024 - 1886호 제 6차 인천시 스포츠산업 창업지원실입
-
교외장학금
23 2024.04[한국장학재단] 2024년 국가우수장학금(인문100년, 예술체육비전) 신규장학생 추가 신청
2024년 한국장학재단 국가우수장학금(인문100년, 예술체육비전) 신규장학생 배정인원이 확
-
모집
22 2024.04[창업지원단] 인천항 두드림 사업(항만,물류,해양,환경,안전) 참여기업 모집 공고
아이엔유파트너스(인천대학교 창업지원단) 공고 제2024 - 101호 「인천항만공사 기업자율
-
일반
22 2024.04[사범대학] 2024학년도 1학기 성인지교육 안내
2024학년도 1학기 성인지교육 안내 성인지교육은 6/29(토), 10/12(토)로 운영
-
행사
22 2024.04학생 맞춤형 AI 교육지원시스템 오픈 및 관련행사(명칭 공모전, 버그 찾기 대회) 안내
학생 맞춤형 AI 교육지원시스템 오픈 및 관련행사(명칭 공모전, 버그 찾기 대회) 안내 폭
-
학사
08 2024.042024년 제2차 평생교육사 자격증 발급 신청 안내
2024년 제2차 평생교육사 자격증 발급 신청 안내 [2024년 제2차 평생교육사 자격증
-
학사
04 2024.04INU 생성형 인공지능(챗GPT) 학습자를 위한 매뉴얼 안내
교육혁신원에서는 생성형 인공지능(챗GPT)의 교육적 활용을 활성화하기 위하여 학습자용 매뉴
-
학사
03 2024.042024학년도 재수강(수기) 신청 안내(~4/5)
2024학년도 1학기 재수강(수기) 신청 안내 1. 관련근거 ○ 인천대학교 학칙 시행세
-
학사
28 2024.03대학수학 튜터시간표
Tutor 시간표 대학수학 (1) 요일월화수목금시간 10:00~12:00/14:00~16:
-
학사
28 2024.032024학년도 1학기 수강포기 처리완료 안내
2024학년도 1학기 수강포기 처리완료 안내 2024학년도 1학기 수강포기 신청에 대한 처
-
학사
27 2024.032024학년도 1학기 부·복수·연계전공 포기 신청 안내
[2024학년도 1학기 부 복수 연계전공 포기신청 안내]2024학년도 1학기 부 복수 연계
-
학점교류
19 2024.042024학년도 하계 계절학기 한양대학교(ERICA) 학점교류 안내(~5/7 13:00)
2024학년도 하계 계절학기 한양대학교(ERICA) 학점교류 안내 1. 신청자격 - 본교
-
학점교류
17 2024.042024학년도 하계 계절학기 강원대학교 학점교류 안내(~4/24 13:00)
2024학년도 하계 계절학기 강원대학교 학점교류 안내 1. 신청자격 - 본교 1학년 이상
-
학점교류
17 2024.042024학년도 하계 계절학기 영남대학교 학점교류 안내(~5/15 13:00)
2024학년도 하계 계절학기 영남대학교 학점교류 안내 1. 신청자격 - 본교 1학년 이상
-
학점교류
11 2024.042024학년도 하계 계절학기 충북대학교 학점교류 안내(~4/18 13:00)
2024학년도 하계 계절학기 충북대학교 학점교류 안내 1. 신청자격 - 본교 1학년 이상
-
학점교류
19 2024.022024학년도 1학기 을지대학교 학점교류 안내(~2/22 13:00)
2024학년도 1학기 을지대학교 학점교류 안내 1. 신청자격 - 본교 1학년 이상 수료한
-
모집
23 2024.04[창업지원단]제 6차 인천시 스포츠산업 창업지원실 입주기업 모집 공고
인천대학교 창업지원단 공고 제2024 - 1886호 제 6차 인천시 스포츠산업 창업지원실입
-
모집
22 2024.04[창업지원단] 인천항 두드림 사업(항만,물류,해양,환경,안전) 참여기업 모집 공고
아이엔유파트너스(인천대학교 창업지원단) 공고 제2024 - 101호 「인천항만공사 기업자율
-
일반
22 2024.04[사범대학] 2024학년도 1학기 성인지교육 안내
2024학년도 1학기 성인지교육 안내 성인지교육은 6/29(토), 10/12(토)로 운영
-
행사
22 2024.04학생 맞춤형 AI 교육지원시스템 오픈 및 관련행사(명칭 공모전, 버그 찾기 대회) 안내
학생 맞춤형 AI 교육지원시스템 오픈 및 관련행사(명칭 공모전, 버그 찾기 대회) 안내 폭
-
모집
19 2024.04★수정/ 2024년도 1학기(1차) 창업장학생 선발 안내문 (~5/6까지)
인천대학교 창업지원단에서는 창업에 관심 있는 (예비)학생 창업자들에게 창업 경험의 기회를
-
모집
19 2024.042024년 제3차 인천대학교 계약직원 채용 공고
2024년 제3차 인천대학교 계약직원 채용 계획을 붙임과 같이 공고하오니 참신하고 역량 있
-
국가근로장학금
23 2024.04[사범대학] 2024학년도 1학기 사범대학 국가근로장학생 추가 모집 안내(~4/29(월))
2024학년도 1학기 사범대학 국가근로장학생을 모집합니다.(1) 모집인원 : 1명(2) 근
-
교외장학금
23 2024.04[한국장학재단] 2024년 국가우수장학금(인문100년, 예술체육비전) 신규장학생 추가 신청
2024년 한국장학재단 국가우수장학금(인문100년, 예술체육비전) 신규장학생 배정인원이 확
-
국가근로장학금
17 2024.04[교무과] 2024학년도 1학기 국가근로장학생 모집 안내(~4/24(수) 23:59까지)
[2024-1학기 교무과 국가근로장학생 모집 안내] 1. 모집인원: 1명 2. 근무조건
-
교내장학금
16 2024.04[교내] 2024-1학기 인천대사랑장학금 신청 안내 2024.04.22(월)~26(금)
2024-1학기 인천대사랑장학금 신청 안내 1. 지원대상장학금지원대상추천장학금성격비고인천대
-
국가근로장학금
09 2024.04[디자인학부] 2024학년도 1학기 국가근로장학생 선발(재공고)
2024-1학기 디자인학부 국가근로장학생 추가 모집(재공고)에 지원해주신 모든 분들께 감사
-
국가근로장학금
08 2024.04[학사팀] 2024-1학기 국가근로장학생 선발 결과(재공고)
국가근로선발결과 학사팀 2024-1학기 국가근로장학생 모집결과를 안내합니다. ○ 재공고에
-
등록금 납부
19 2024.042024학년도 1학기 재학생 추가 등록 기간 시행 안내(최종)
2024-1학기 재학생 최종 등록 기간을 다음과 같이 안내 하오니, 미등록자는 기한 내에
-
등록금 납부
12 2024.042024-1학기 재학생 등록금 납부 기간 연장 안내(8차)
2024-1학기 재학생 등록 기간을 다음과 같이 연장(8차) 하오니, 미등록자는 기한 내에
-
등록금 납부
05 2024.042024-1학기 재학생 등록금 납부 기간 연장 안내(7차)
2024-1학기 재학생 등록 기간을 다음과 같이 연장(7차) 하오니, 미등록자는 기한 내에
-
등록금 납부
29 2024.032024-1학기 재학생 등록금 납부 기간 연장 안내(6차)
2024-1학기 재학생 등록 기간을 다음과 같이 연장(6차) 하오니, 미등록자는 기한 내에
-
등록금 납부
22 2024.032024-1학기 재학생 등록금 납부 기간 연장 안내(5차) 및 8학기 경과자 등록 안내
2024-1학기 재학생 등록 기간을 다음과 같이 연장(5차) 하오니, 미등록자는 기한 내에
-
교육시험
22 2024.022024년 4월 21일 HSK/HSKK 정기시험 접수안내
[2024년 4월 21일 HSK/HSKK 정기시험 접수안내] 1. 접수기간 : 2024.3
-
교육시험
22 2024.022024년 HSK/HSKK 시험 일정
□ 2024년 HSK 및 HSKK 시험 일정연 번시험 종류시험 일자신청 마감일성적 조회일1
-
교육시험
18 2023.102023년 12월 3일 HSK/HSKK 정기시험 접수안내
[2023년 12월 3일 HSK/HSKK 정기시험 접수안내] 1. 접수기간 : 2023.
-
교육시험
10 2023.102023년 11월 18일 HSK/HSKK 정기시험 접수안내
[2023년 11월 18일 HSK/HSKK 정기시험 접수안내] 1. 접수기간 : 2023
-
교육시험
24 2023.082023년 10월 15일 HSK 정기시험 접수안내
[2023년 10월 15일 HSK 정기시험 접수안내] 1. 접수기간 : 2023.9.18.
-
봉사
19 2024.04[사회봉사센터] 2024년 하계 INU글로벌사회공헌 봉사단원 모집 안내
[사회봉사센터] 2024년 하계 INU글로벌사회공헌 봉사단원 모집 안내 *첨부파일(1.홍보
-
봉사
18 2024.04[사회봉사] (인화여자고등학교) 교육멘토링 봉사자 모집
[인화여자고등학교] 교육멘토링 봉사자 모집2024 인화여자고등학교 교육복지우선지원사업도란도
-
봉사
12 2024.04[사회봉사] (한국우편사업진흥원) 우정사회봉사단 모집
2024년 대학생 우정사회봉사단 포커스:온 5기 모집1. 대상: 2024년도 1~2학기 전
-
봉사
11 2024.04[사회봉사] (만월종합사회복지관) 어린이날 자원봉사 모집
-
봉사
08 2024.04[사회봉사] (부평중부종합사회복지관) 텃밭조성사업 봉사자 모집
-
모집
28 2023.07[기초과학연구소] 기초학문 박사후연구원 모집공고
인천대학교 기초학문분야 박사후(PostDoc.) 연구원 채용공고 인천대학교 기초
-
모집
28 2023.07[인천국제개발협력센터] 2023 ODA 전문인력교육과정 <모니터링과 평가(M&E)> 수강생
안녕하세요? 인천국제개발협력센터입니다.인천국제개발협력센터는 인천 지역의 국제개발협력 사업
-
모집
27 2023.07[공학교육혁신센터] 2023 수소 및 연료전지 분야 직무교육 참가학생 모집(~08.16)
성균관대학교 공학교육혁신센터에서 주관하는 한국신재생에너지협회와 함께하는 「수소 연료전지 분
-
모집
26 2023.07[국제교류팀] 해외인턴 프로그램 설명회 개최안내
국제교류팀에서는 2023년도 하반기 해외인턴 프로그램 설명회를 아래와 같이 개최하오니 학생
-
모집
24 2023.07[창업지원단] 23년도 2학기 창업대체 학점인정제 모집공고(~8.8.(화) 24시까지)
-
모집
21 2023.07[공학교육혁신센터] 2023-2 메이커스페이스 근로 장학생 모집
공학교육혁신센터 메이커스페이스에서 2023년도 2학기 근로 장학생을 모집합니다.관심 있는
- 최근게시물이(가) 없습니다.
-
미추홀캠퍼스
- 인천광역시 연수구 갯벌로 12 (우 21999)
- #동북아물류 E-Biz센터 #미래관 #미추홀 별관 A동 #미추홀 별관 B동 #INU 이노베이션센터
-
제물포캠퍼스
- 인천광역시 미추홀구 석정로 165 (우 22100)
- #성지관



















 INSTAGRAM
INSTAGRAM
 NAVER
NAVER
 YOUTUBE
YOUTUBE
 FACEBOOK
FACEBOOK











